News
Jordan Berman, Vice President, Global Corporate Affairs
Tel: 1 (416) 749-9026 Ext. 7487
E-Mail: jberman@apotex.com
FOR IMMEDIATE RELEASE – March 1, 2023 – Weston, Florida, Apotex Corp., with the knowledge of the US FDA, is initiating a voluntary recall at the Consumer level for six (6) lots of Brimonidine Tartrate Ophthalmic Solution, 0.15% specified below. This recall is being initiated out of an abundance of caution due to cracks that have developed in some of the units caps of Brimonidine tartrate ophthalmic solution bottles. There is a possibility the broken cap may impact sterility and if so, the possibility of adverse events.
Brimonidine Tartrate Ophthalmic Solution is an alpha-adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
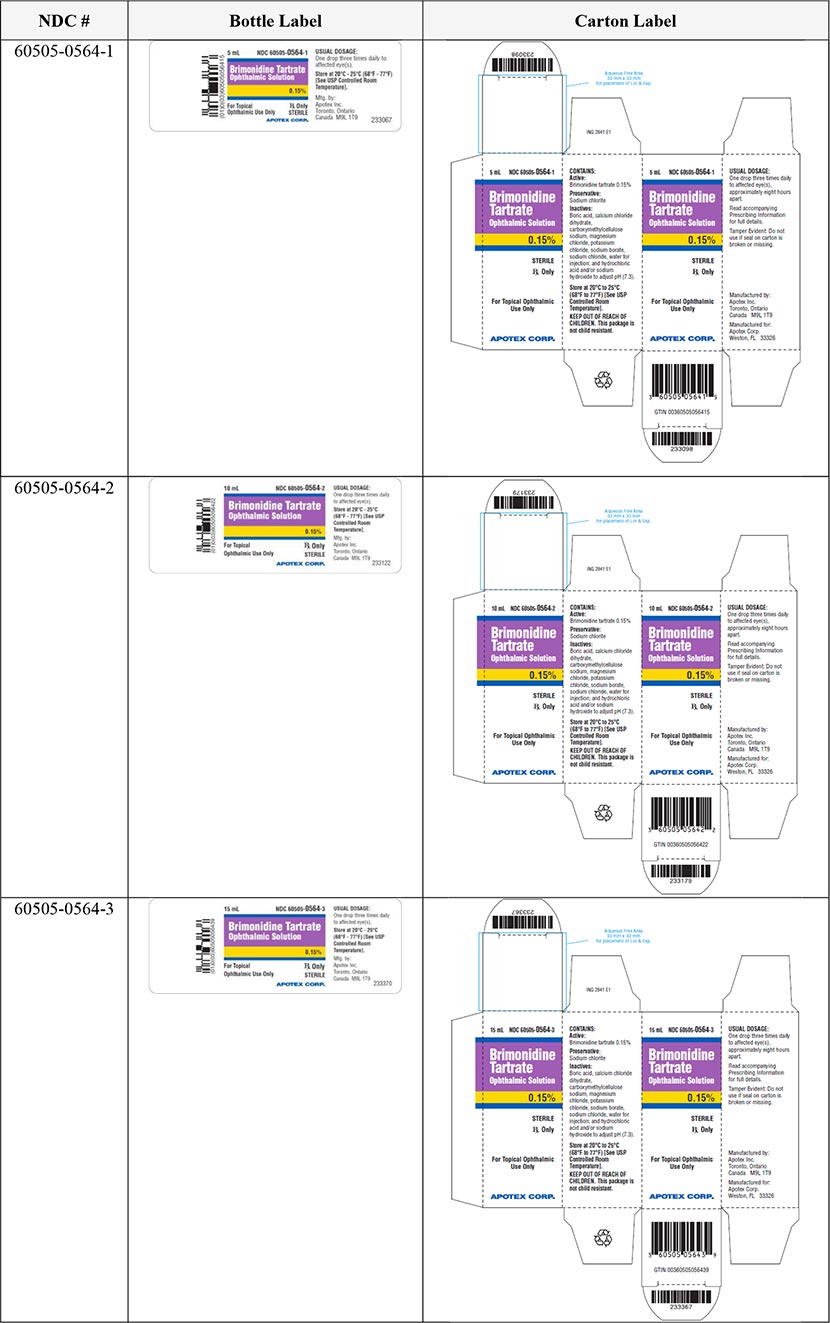
The six (6) lots of Brimonidine Tartrate Ophthalmic Solution, 0.15% can be identified by NDC numbers stated on the carton and label of the product. The lot number and expiry date are located on the top flap of the carton and to the left side of the product description on the bottle label beside the barcode. These lots were distributed nationwide in the USA between April 05, 2022 to February 22, 2023.
|
Product |
Strength |
Pack Size |
NDC # |
UPC Code |
UPC Code |
Lot # |
Expiry Date |
|---|---|---|---|---|---|---|---|
|
Brimonidine Tartrate Ophthalmic Solution |
0.15% |
5 mL |
60505-0564-1 |
360505056415 |
(01)0(03) |
TJ9848 |
02/2024 |
|
TJ9849 |
|||||||
|
TK0258 |
04/2024 |
||||||
|
TK5341 |
|||||||
|
10 mL |
60505-0564-2 |
360505056422 |
(01)0(03) |
TK0261 |
|||
|
15 mL |
60505-0564-3 |
360505056439 |
(01)0(03) |
TK0262 |
Apotex Corp. is notifying all impacted direct accounts (Wholesalers, Distributors, Warehousing Chains, Mail Order Pharmacy and Long-Term Care Pharmacy) of this voluntary recall via email and mail (FedEx Standard Overnight) and is arranging for return of all recalled product.
Patients who have received the identified lots or have questions regarding this recall should contact their pharmacy. They should immediately contact their health care provider for medical advice and return the identified lots to Inmar Rx Solutions by contacting at the phone number provided in this press release.
Wholesalers, Distributors, Warehousing Chains, Mail Order Pharmacy and Long-Term Care Pharmacy should return the recalled product to the place of purchase. Anyone with an existing inventory of the recalled product should quarantine the recalled lots immediately. Customers who purchased the impacted product directly from Apotex Corp. can call Inmar Rx Solutions at 1-855-275-1273 (9:00am – 5:00pm, EST Monday thru Friday), to arrange for their return.
Consumers with the impacted units of Brimonidine Tartrate Ophthalmic Solution, 0.15%, can contact Inmar Rx Solutions at 1-855-275-1273, to receive a recall/return packet including the Recall Stock Response Form (or you may obtain this form from clsnetlink.com)
Consumers with questions regarding this recall can contact Apotex Corp. by phone at 1-800-706-5575 (8:30am – 5:00pm, EST Monday thru Friday) or email address UScustomerservice@Apotex.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Product Label
-- # --
